Deallus is a unique strategic intelligence consultancy operating across the global life sciences sector
We offer a broad range of career opportunities, dependent on your life sciences experience
We have a number of global offices and we’re ready and waiting to talk about how we can assist you with your project
We are a company of experienced and dedicated life sciences specialists. Our unparalleled competitive intelligence capabilities are enhanced by the strategic mindset that drives our entire organization.
This enables us to provide you with the assured guidance you need to make the right strategic decisions. We are well equipped to assist with any issue at any stage of your product’s lifecycle.
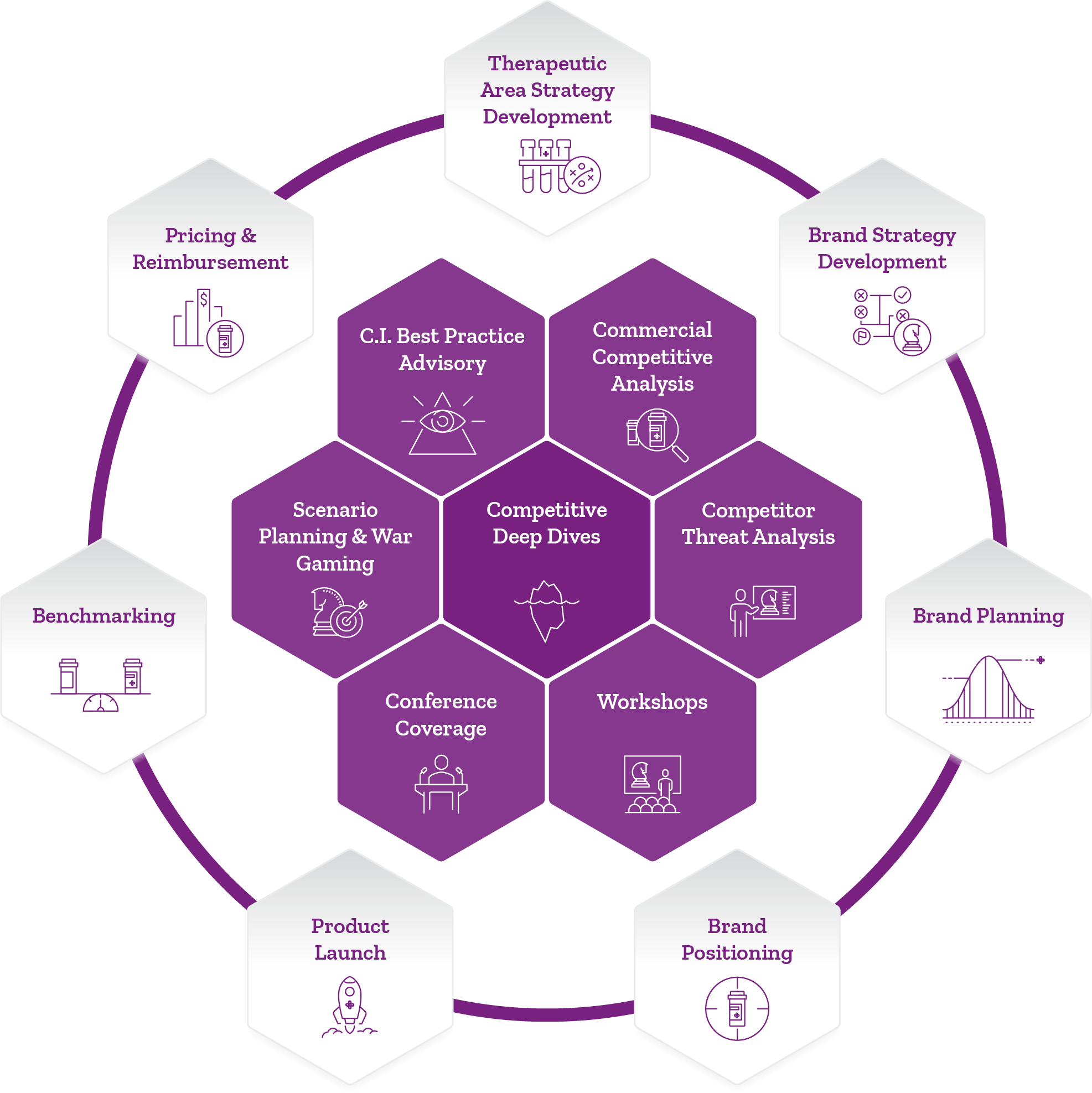
Therapeutic Area Strategy Development
-
Clinical pathway design
-
Trial design
-
BD&L/Partnering Strategy
-
Portfolio prioritization
Brand Strategy Development
-
Forecasting
-
Stakeholder selection & engagement
-
Market Research
-
Message development
-
Patient journey
Brand Planning
-
Strategic/tactical plan development
-
Forecasting
-
Budgeting & tactics
-
Customer segmentation
-
Brand planning process support/PMO
Brand Positioning
-
Brand attribute testing
-
Differentiation
-
Positioning research
-
Training plan
Product Launch
-
Launch plan development
-
Launch office set-up & day-to-day operation
-
Launch readiness assessment
-
Progress reviews & tracking
Benchmarking
-
Analogue analysis
-
Best practices
-
Key performance drivers
-
Competitive capabilities
Pricing & Reimbursement
-
Payer landscape assessment
-
Pricing strategy
-
Patient support
-
Contracting strategy
-
Payer segmentation
Competitive Deep Dives
Competitive profiling of multiple potential sources of competitive advantage
-
TPP
-
Commercial capabilities
-
Regulatory/government affairs set-up
-
Pricing reimbursement approach
-
Value-added services
-
Field stakeholder engagement
Commercial Competitive Analysis
Studies on competitors:
-
Sales teams
-
Account teams
-
Commercial digital approach
-
Product positioning/messaging
Competitor Threat Analysis
Detailing competitor:
-
Strengths & weaknesses
-
Decision-making timelines
-
Commitments
-
Strategic intent
Workshops
-
Alignment workshops
-
Discovery workshops
-
Competitive insights workshops
Conference Coverage
-
In-depth coverage of presentations & booth displays
-
Expert commentary at industry conferences worldwide
Scenario Planning & War Gaming
-
Role plays
-
Simulations
-
What-if/contingency planning
-
Communications roadmap building
C.I. Best Practice Advisory
-
Boot camps
-
Masterclasses
-
Organization design for world-class C.I. functions
Can AI really help you understand what matters in your market?
The impact of AI across our industry will be profound. It’s already driving faster identification of novel drug targets and drug discovery, better patient identification, accelerated drug development, earlier diagnosis, and more personalized healthcare.
Brain Awareness Week
Brain Awareness Week is a global campaign held every March that aims to increase public support and excitement for brain science.
Rare Diseases, Pharma’s Perennial Hitmaker
February 29th is the rarest day of the year, and this was in fact the reason why Rare Disease Day was established on this day 16 years ago by the European Organisation for Rare Diseases, commemorating the 25th anniversary of the U.S. Orphan Drug Act.
National Healthcare Security Administration (NHSA) protocols
On 4 July 2023, the National Healthcare Security Administration (NHSA) published the drafted 'Protocol for Renewal of NRDL Listed Drugs’ and ‘Negotiation Rules for Non-Exclusive Drug’. The two protocols aim to streamline the negotiation process for NRDL listed drugs and those with generic versions and are eligible for NRDL listing.
China Pharma Market Access (CPMA)
Since the establishment of the China National Medical Insurance Bureau in 2018, the goal has been to strengthen public health and medical service levels, achieve maximum utilisation of the national medical insurance fund, and to enhance the strategic purchasing capability of medical insurance funds. Significant tools in achieving this are the National Reimbursement Drug List (NRDL) and drug Volume Based Procurement (VBP), which have opened a new chapter in China’s medical reform. Meanwhile, the new drug registration process is improved, simplified, and accelerated.
George Gu attends DIA meeting in Suzhou
George Gu, Principal and Head of China at Deallus, recently attended the 2023 DIA China Annual Conference where he participated in an expert panel discussion on "Post-Marketing Medical Activities Based on Specialty Drug Pharmacy Management Platform".